EXPERIMENT
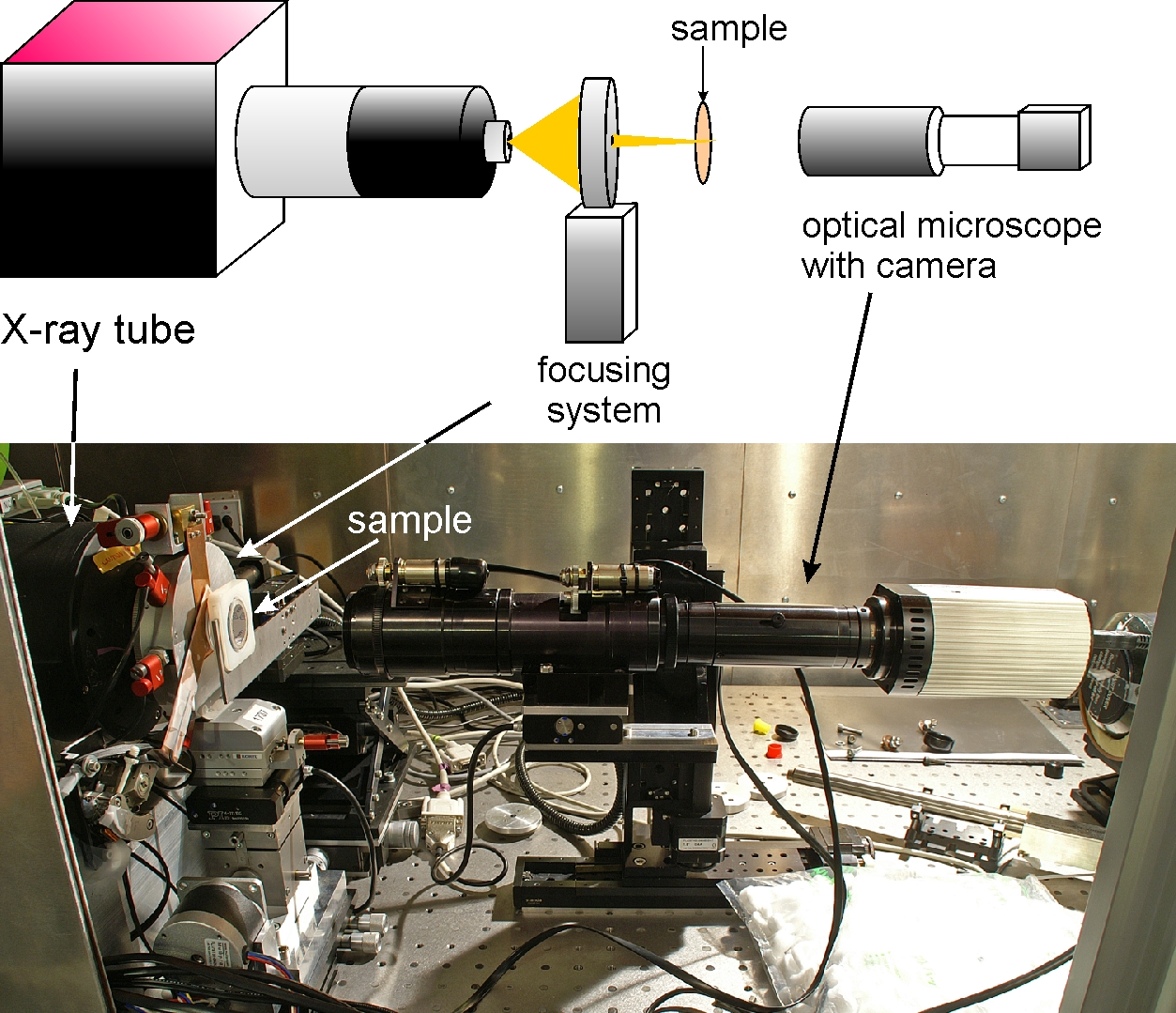
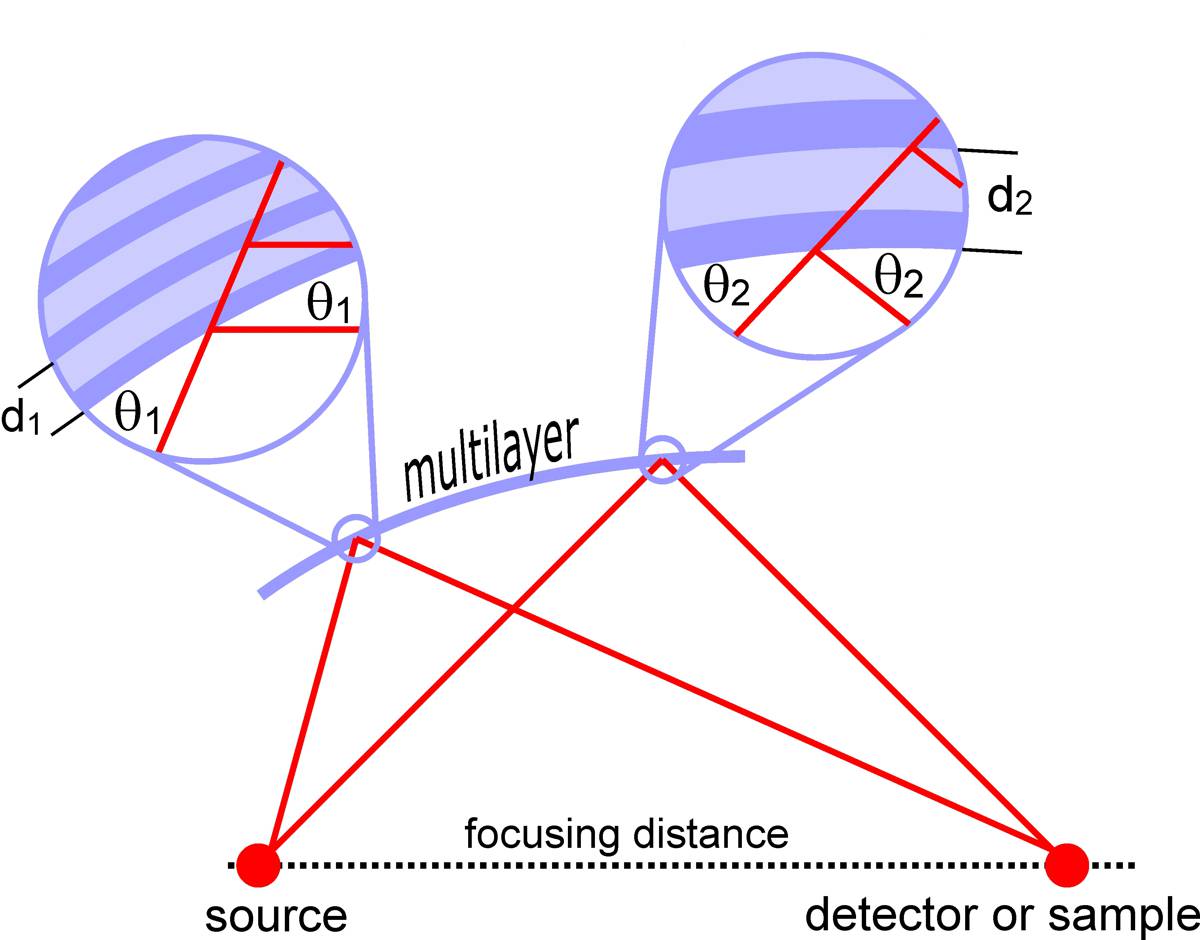
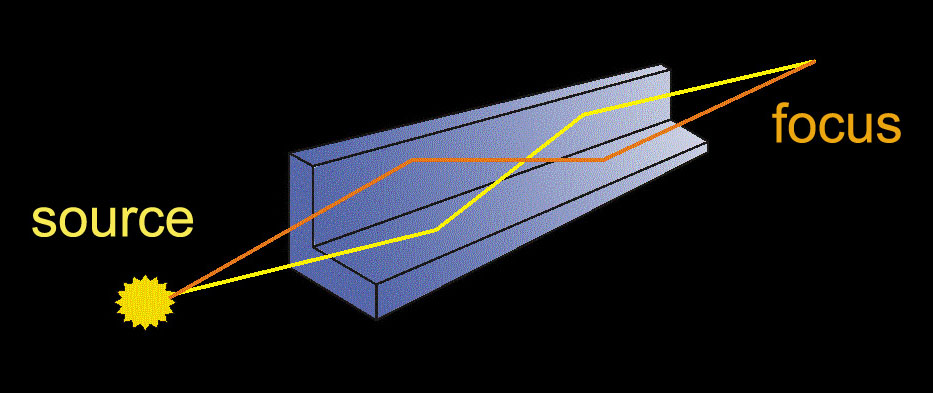
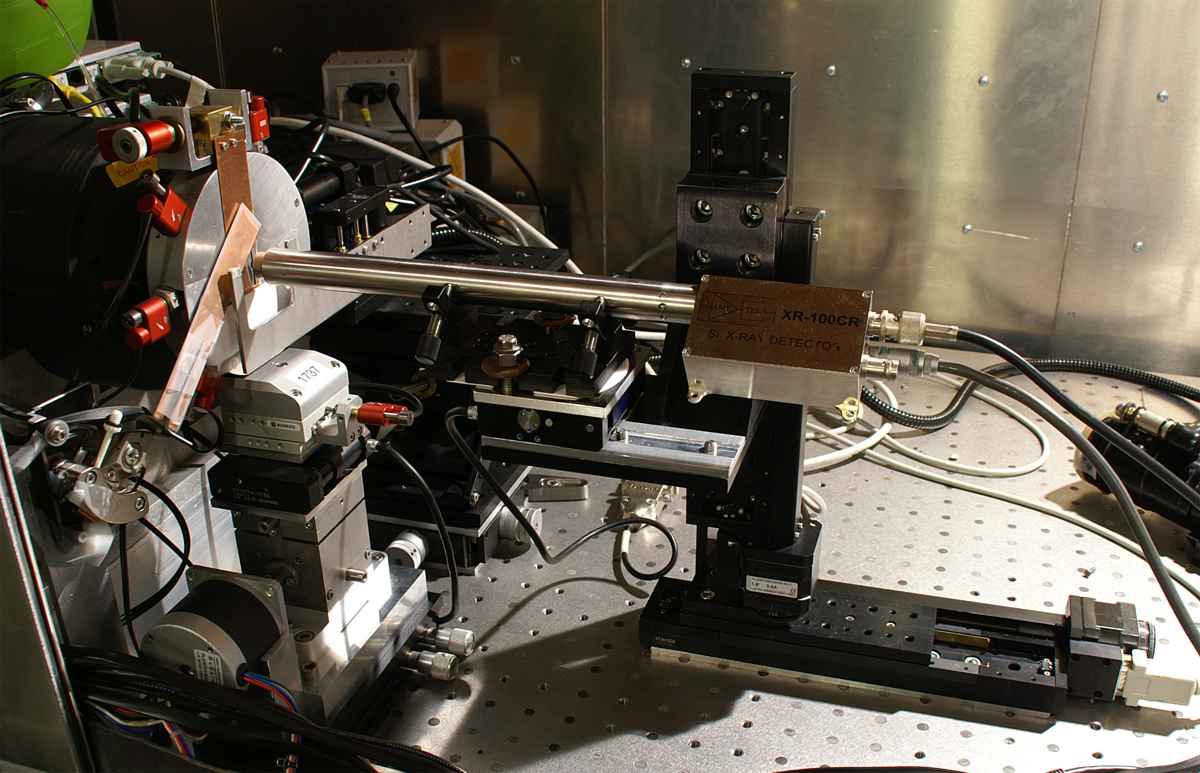
A conical beam of radiation from an X-ray tube coming from an area with a diameter of about 2 µm is focused on the sample by the focusing system. The optical microscope provides a view of the sample, so that the cells dedicated for irradiation can be marked. Two stepper motors move the sample in the plane of focus of the beam. The copper shutter ensures precise exposure time.
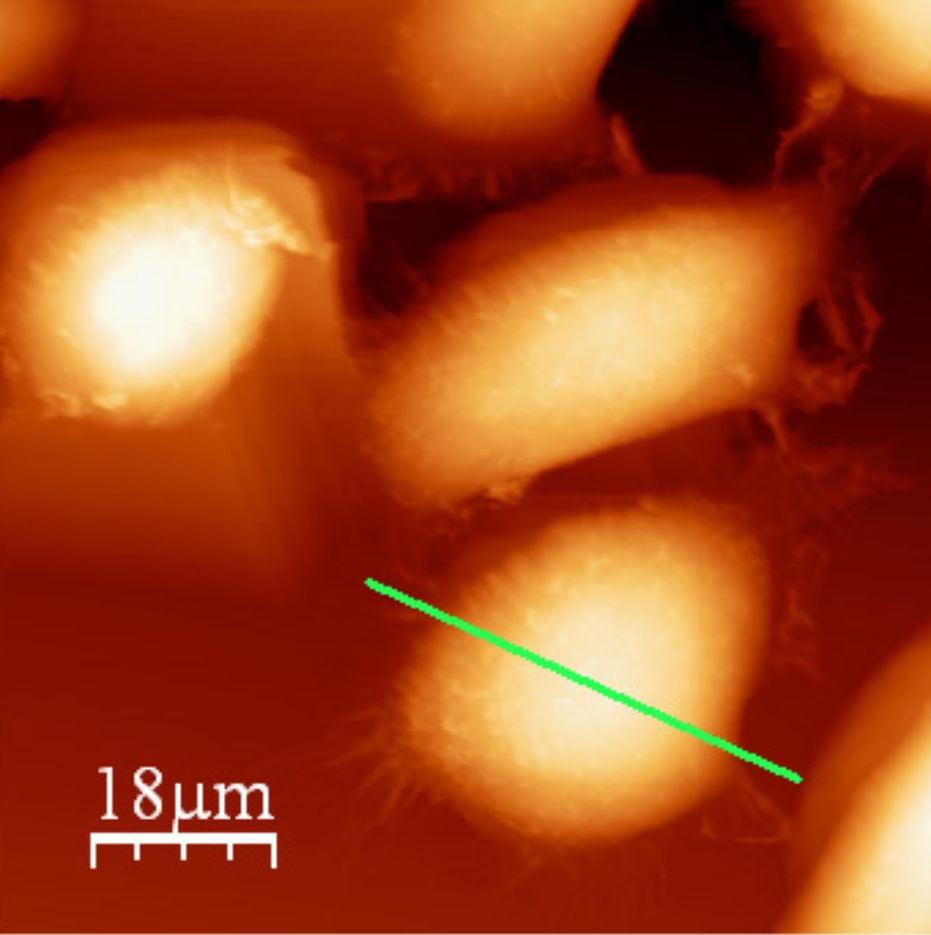
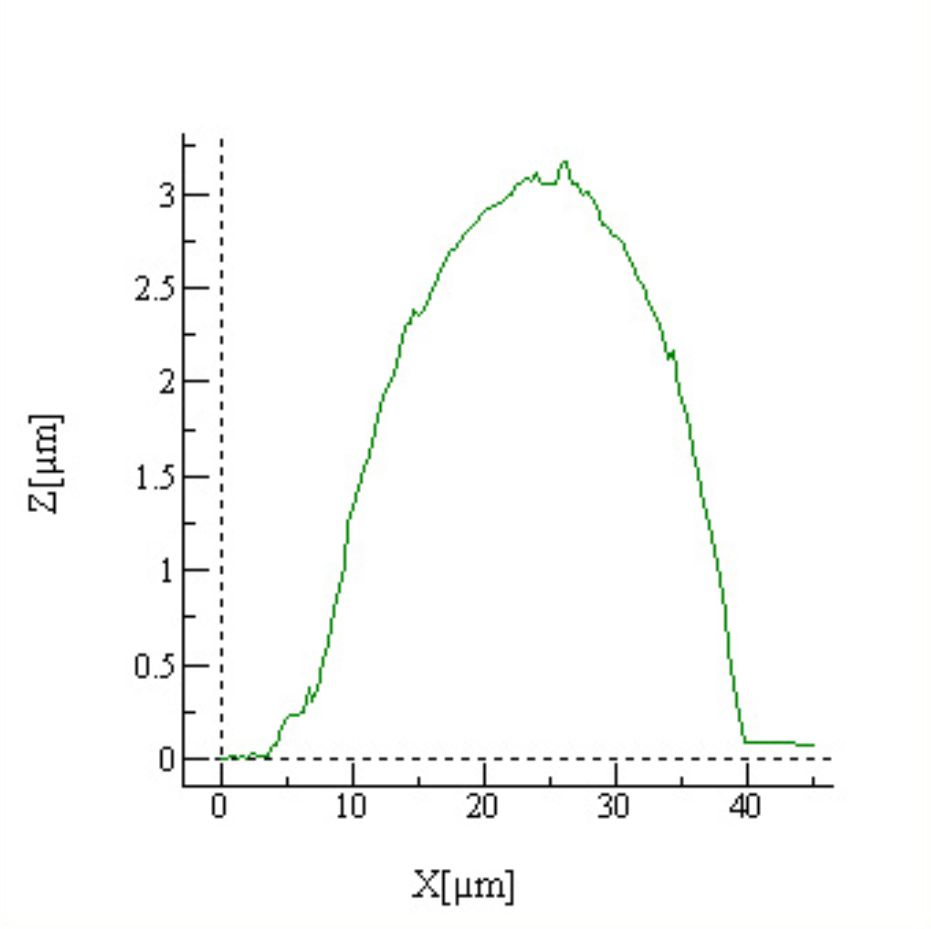
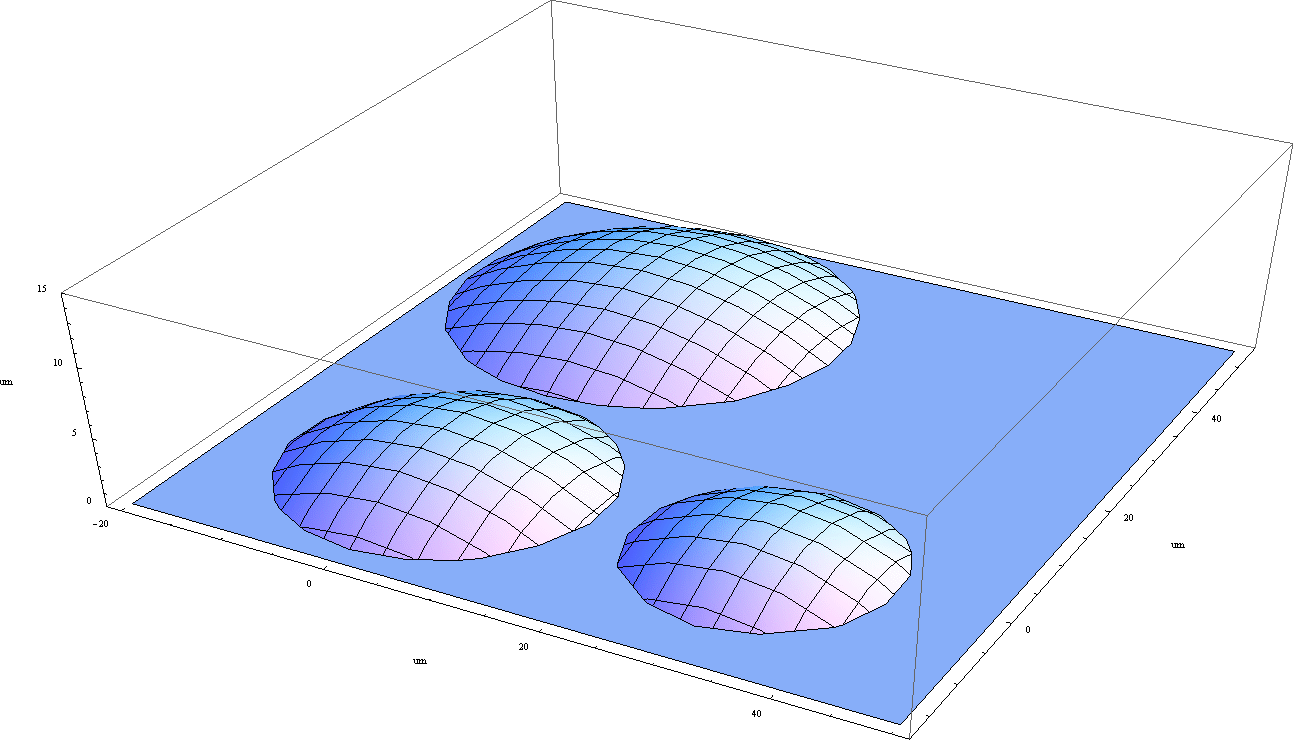
The PC3 cells are seeded on the Mylar foil in a hole drilled in the Petri dish.

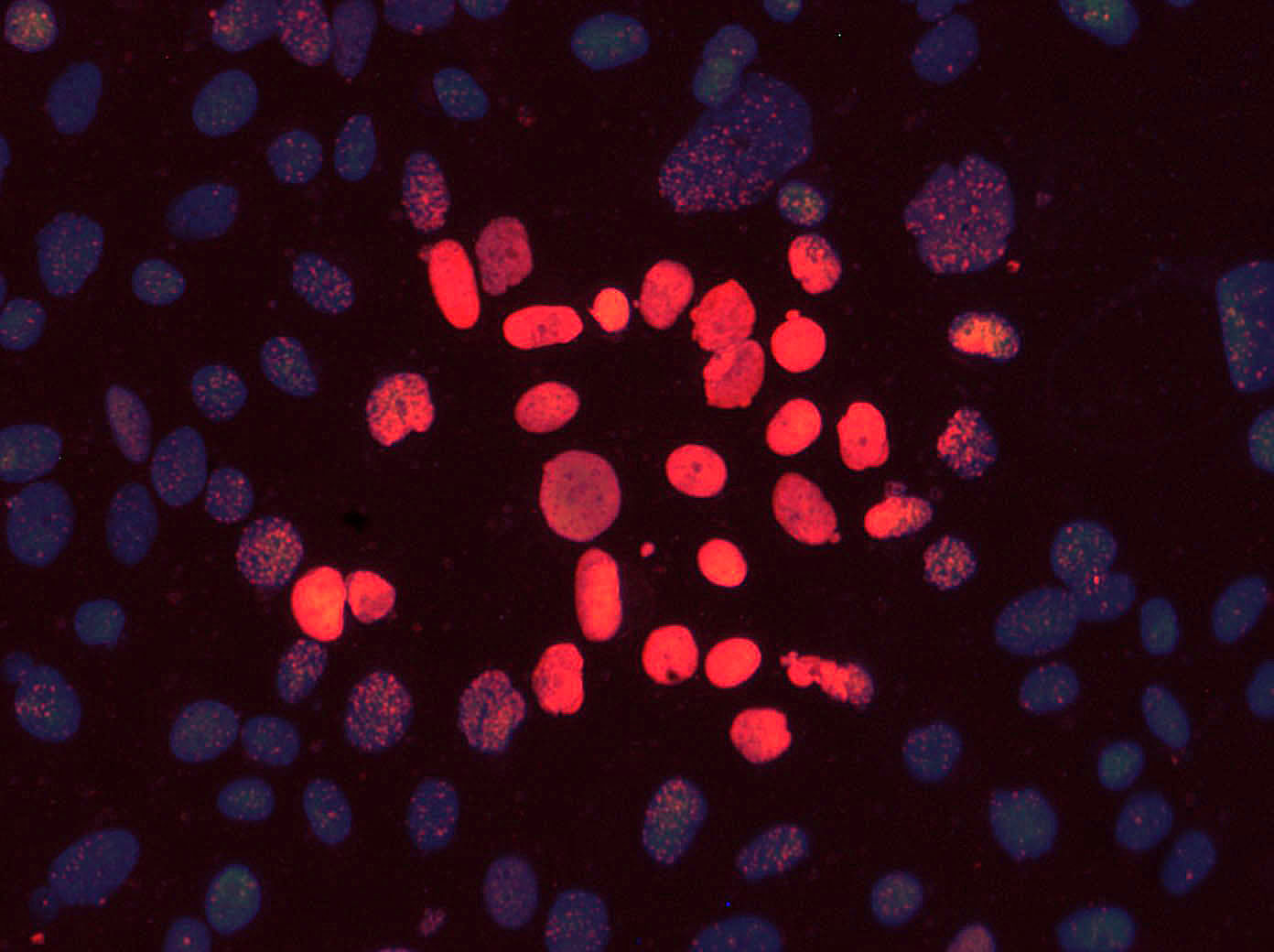
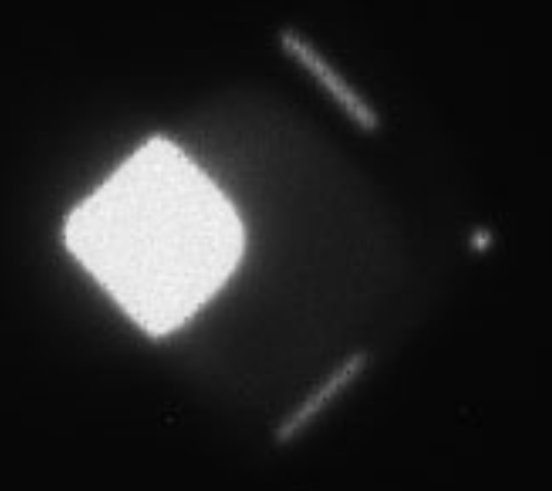
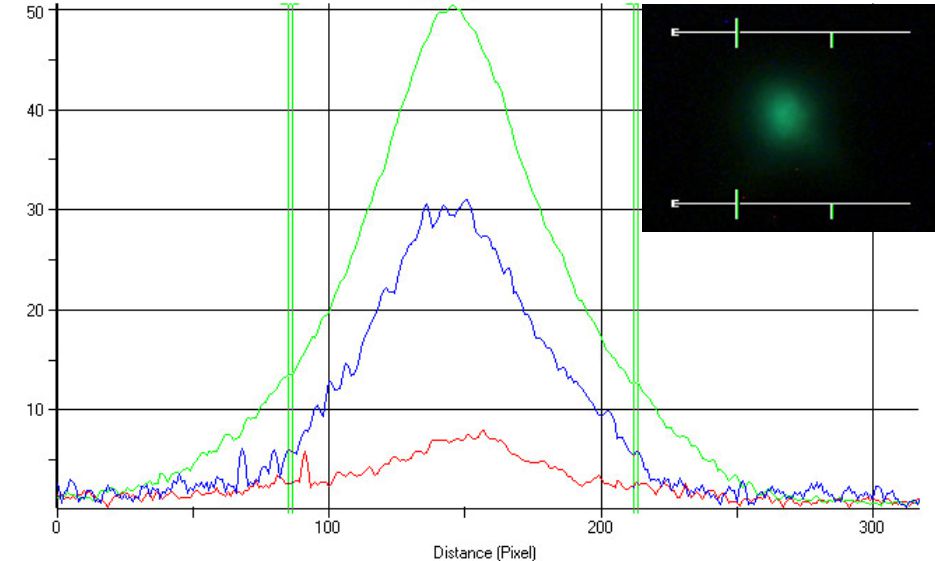
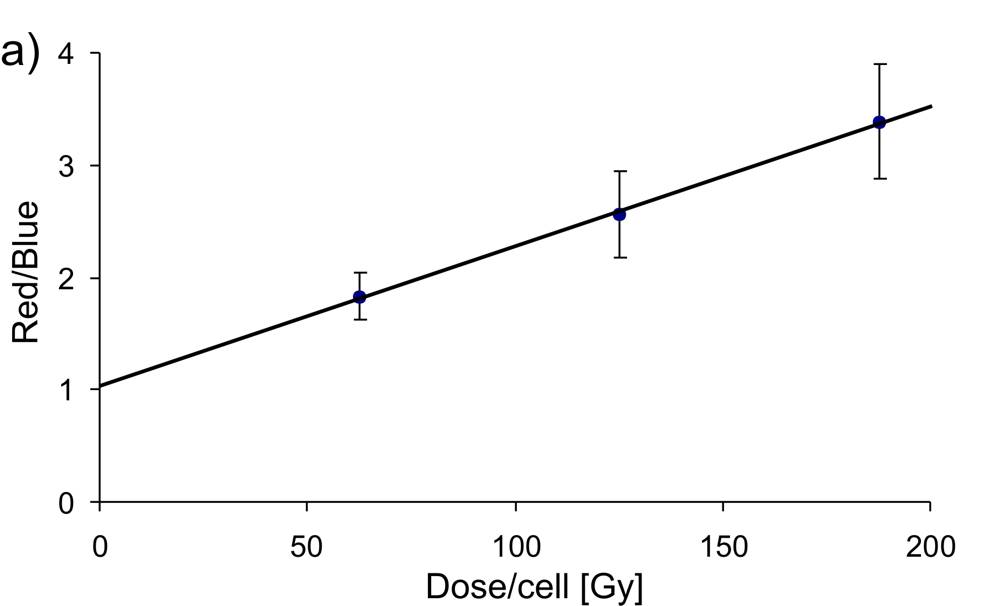
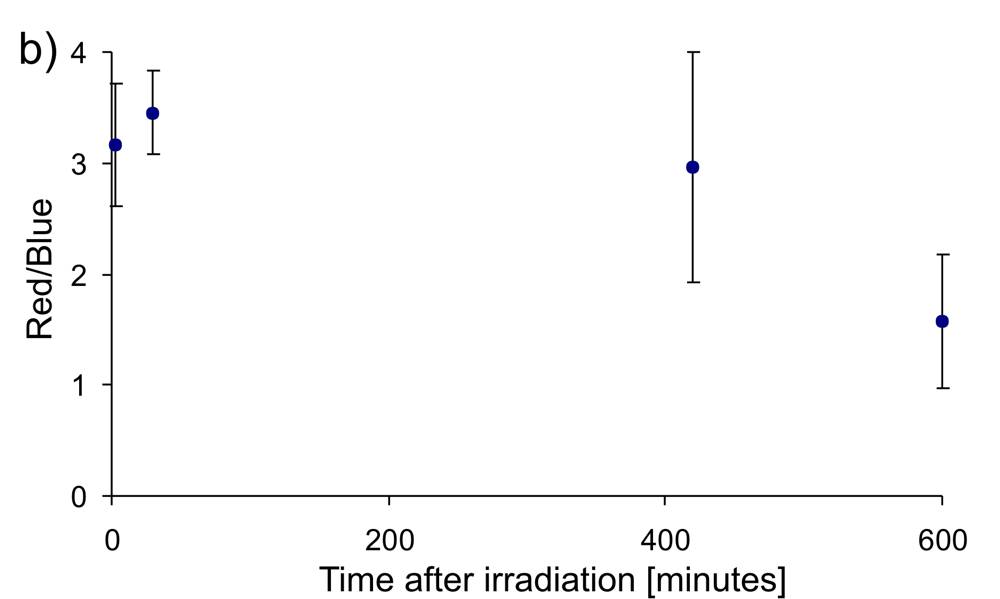
After irradiation, the cells are examined. For the visualization of all cells, the fluorescent dye DAPI (4',6-diamidino-2-phenylindole) is used, which can also penetrate intact cell membranes and binds to the DNA of the cells. When excited by UV light, DAPI emits blue light. On the other hand, Alexa Fluor 488 dye is used to visualize double DNA damage in cells, which glows red when excited with 488 nm light. The overlay of these two images creates visualization of cell nuclei with DNA damages.